[Announcement] HGPI Endorsed “Joint Statement on the Implementation of Comprehensive Genomic Profiling test” (December 15, 2023)
date : 12/21/2023
Tags: NCDs, Precision Cancer Medicine
![[Announcement] HGPI Endorsed “Joint Statement on the Implementation of Comprehensive Genomic Profiling test” (December 15, 2023)](https://hgpi.org/en/wp-content/uploads/sites/2/ncd-pcm-20231215-top.png)
On December 15, 2023, Health and Global Policy Institute (HGPI) endorsed “Joint Statement on the Implementation of Comprehensive Genomic Profiling”.
In addition to our Organization, this Joint Statement is endorsed by the Japan Federation of Cancer Patient Groups, the European Federation of Pharmaceutical Industries and Associations (EFPIA Japan), the Pharmaceutical Research and Manufacturers of America (PhRMA), the Japanese Society of Medical Oncology, the Japanese Society of Clinical Oncology, the Japanese Cancer Association, and the Social Insurance Union of Societies Related to Internal Medicine.
This joint statement will be submitted to the Minister of Health, Labour and Welfare, Mr. Keizo Takemi, and other relevant officials.
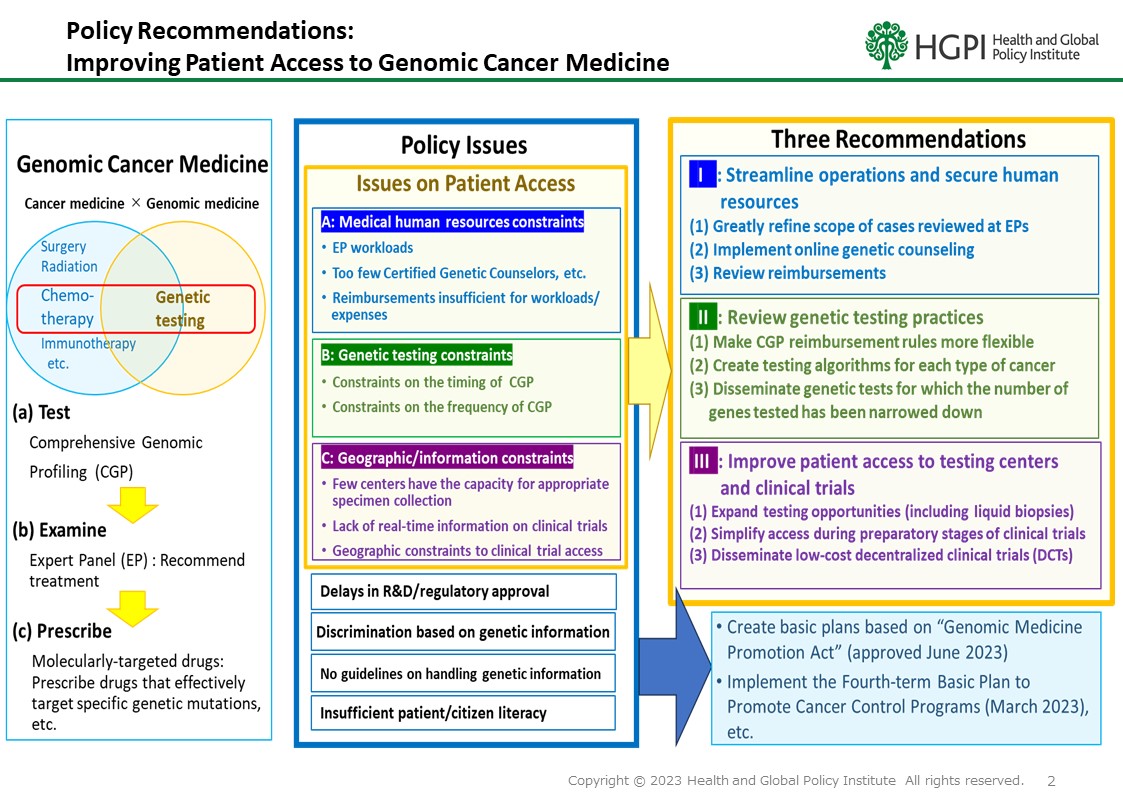
Currently, the cancer gene panel tests (cancer Comprehensive Genomic Profiling tests) approved for use under the Japanese Health insurance system is only for ” solid tumors for which no standard treatment is available, or with local progression or metastasis for which standard treatment has been terminated (including those for whom termination is expected)”. Under these circumstances, there have been unfortunate cases in which patients have missed treatment opportunities due to deterioration of their general condition, etc., even when a cancer gene panel tests has revealed a gene mutation that can be treated with drug therapy, and it is too late to do so. In order to ensure that patients who need the most appropriate treatment can receive it at the most appropriate time, a number of proposals have been made by related organizations to resolve issues related to insurance reimbursement. We share a strong awareness of these issues and strongly request that the following points in particular be implemented in the FY2024 revision of the medical service fee.
Request
Expand insurance coverage for cancer gene panel testing so that it can be done at the appropriate time from the time of initial treatment.
Please note that the joint statement documents are only available in Japanese.
日本語
Top Research & Recommendations Posts
- [Announcement] A Turning Point Towards Building Green Healthcare Systems (June 5, 2024)
- [Policy Recommendations] Developing a National Health and Climate Strategy for Japan (June 26, 2024)
- [Publication Report] Guidance on Patient and Public Involvement (PPI) in Health Policymaking: Necessary Initiatives and Good Examples from the Public and Government (March 31, 2024)
- [Policy Recommendations] Obesity Control Promotion Project 2023 “The Next Steps for Engaging and Cooperating with Patients, Citizens, and Communities for Implements of Obesity Control Measurements” (April 8, 2024)
- [Research Report] Healthcare DX Project Research Report of Interviews ”Expectations for the Coming Era of Healthcare DX from People Living with Health Concerns” (June 10, 2024)
- [Research Report] Building a Mental Health Program for Children and Measuring its Effectiveness (June 16, 2022)
- [Announcement] A Significant Step Towards the Building a Green Healthcare System: Support for the Formal Expression of Interest by the Japanese Government Delegation to the ATACH at the Executive Board Meeting of the WHO (February 16, 2024)
- [Policy Recommendations] Recommendation for the Basic Policy on Economic and Fiscal Management and Reform 2024 (June 11, 2024)
- [Policy Recommendations] Patient and Public Involvement (PPI) Support Project “Promoting PPI in the Policymaking Process” (May 14, 2024)
- [Public Comment Submission] “The Fifth Fundamental Plan for Establishing a Sound Material-Cycle Society (Draft)” (May 22, 2024)
Featured Posts
-
2024-06-21
[Event Report] HGPI Special Seminar – HGPI Celebrates its 20th Anniversary: Reflecting on HGPI’s Journey from the Past to the Future (January 16, 2024)
![[Event Report] HGPI Special Seminar – HGPI Celebrates its 20th Anniversary: Reflecting on HGPI’s Journey from the Past to the Future (January 16, 2024)](https://hgpi.org/en/wp-content/uploads/sites/2/240116_HGPISeminar_eyecatch-1.png)
-
2024-06-25
[Public Comment Submission] Web based consultations on NCDs and mental health by World Health Organization (June 25, 2024)
![[Public Comment Submission] Web based consultations on NCDs and mental health by World Health Organization (June 25, 2024)](https://hgpi.org/en/wp-content/uploads/sites/2/ncd-ppi-ph-mh-20240625-top.png)
-
2024-06-26
[Policy Recommendations] Developing a National Health and Climate Strategy for Japan (June 26, 2024)
![[Policy Recommendations] Developing a National Health and Climate Strategy for Japan (June 26, 2024)](https://hgpi.org/en/wp-content/uploads/sites/2/NHCSJ_ENG.png)
-
2024-06-26
[Registration Open] (Webinar) The 127th HGPI Seminar: Current Issues and Future Prospects in Establishing a Health System and Protecting Public Health Through Policy (July 18, 2024)
![[Registration Open] (Webinar) The 127th HGPI Seminar: Current Issues and Future Prospects in Establishing a Health System and Protecting Public Health Through Policy (July 18, 2024)](https://hgpi.org/en/wp-content/uploads/sites/2/HGPI_20240718_127thHGPISeminar.png)
-
2024-07-01
[Registration Open] Meaningful Involvement Promotion Project Online Expert Meeting “Shaping the Future of Health Policy with People with Lived Experience and Citizens” (July 26, 2024)
![[Registration Open] Meaningful Involvement Promotion Project Online Expert Meeting “Shaping the Future of Health Policy with People with Lived Experience and Citizens” (July 26, 2024)](https://hgpi.org/en/wp-content/uploads/sites/2/HGPI_20240726_Shaping-the-Future-of-Health-Policy-with-People-with-Lived-Experience-and-Citizens.jpg)